


石油化工高等学校学报 ›› 2024, Vol. 37 ›› Issue (6): 1-12.DOI: 10.12422/j.issn.1006-396X.2024.06.001
收稿日期:2024-08-03
修回日期:2024-09-25
出版日期:2024-12-25
发布日期:2024-12-24
通讯作者:
尤雅
作者简介:贺东玮(1997-),男,博士研究生,从事钠离子电池层状氧化物方面的研究;E-mail:303439@whut.edu.cn。
基金资助:
Dongwei HE1( ), Bin ZHANG2, Ya YOU1(
), Bin ZHANG2, Ya YOU1( )
)
Received:2024-08-03
Revised:2024-09-25
Published:2024-12-25
Online:2024-12-24
Contact:
Ya YOU
摘要:
钠离子层状氧化物是钠离子电池最具潜力的正极材料之一,具有高容量、低成本等优势,因此钠离子电池在大规模静态储能领域有巨大的应用前景。但是,较差的电化学循环稳定性和空气稳定性使其商用化发展受到限制。相比于传统的多晶层状氧化物,单晶层状氧化物具有高机械强度、低比表面积、高压实密度等特点,因此能有效地改善层状氧化物的循环稳定性,提高其综合性能。介绍了钠离子电池层状氧化物的基本结构类型;回顾了目前已经报道的钠离子电池单晶层状氧化正极的合成方法,并分析了各种合成方法的优劣势;阐述了单晶形貌对于钠离子层状氧化物综合性能的提升机理,以及钠离子电池单晶层状氧化物的研究现状,并对未来钠离子电池单晶层状氧化物的发展进行了展望。
中图分类号:
贺东玮, 张彬, 尤雅. 钠离子单晶层状氧化物正极研究进展[J]. 石油化工高等学校学报, 2024, 37(6): 1-12.
Dongwei HE, Bin ZHANG, Ya YOU. Research Progress of Sodium Ion Single-Crystal Layered Oxides[J]. Journal of Petrochemical Universities, 2024, 37(6): 1-12.
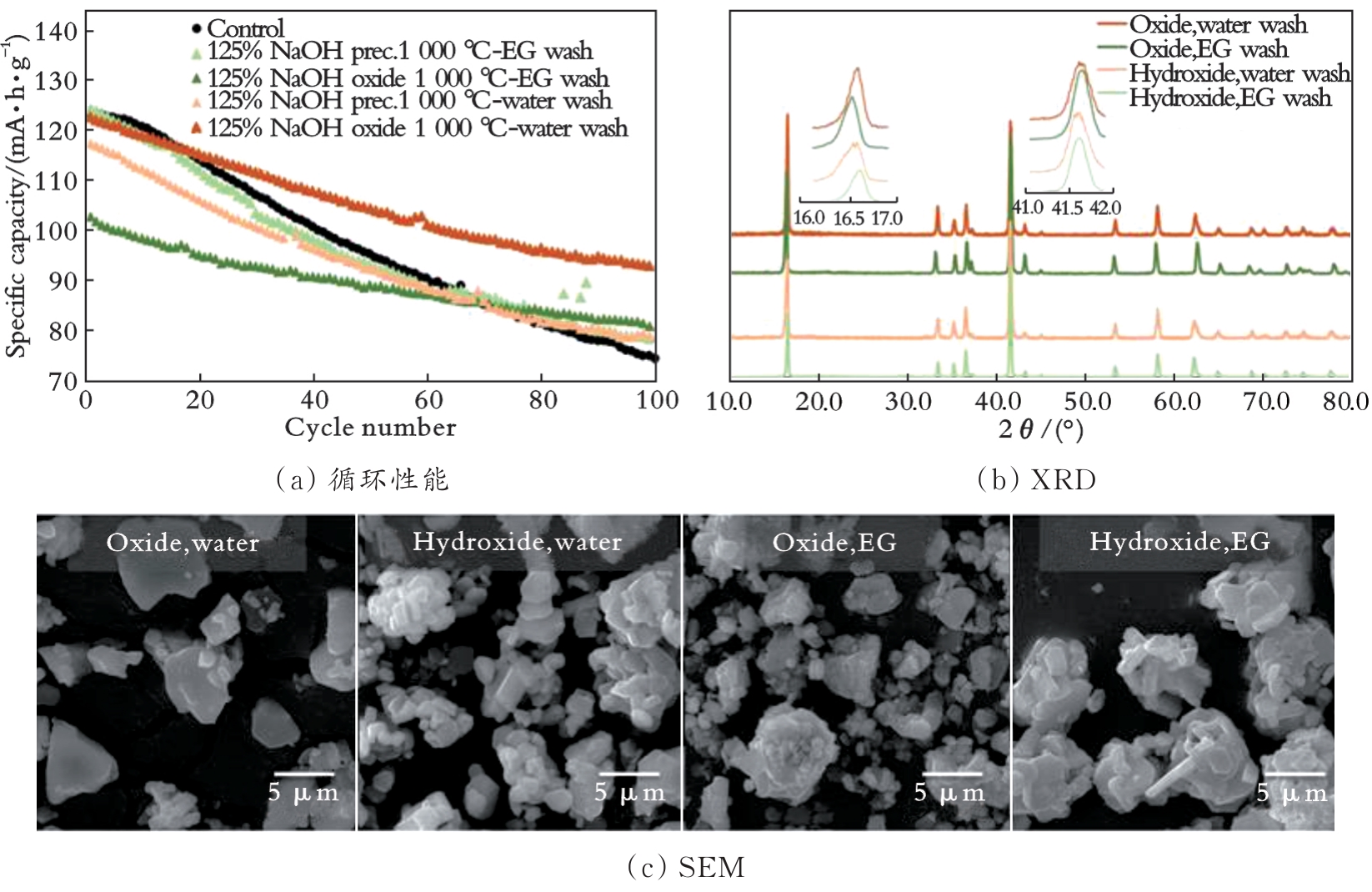
图3 乙二醇或水洗后单晶NaNi0.3Fe0.4Mn0.3O2的循环性能、XRD和SEM[52]
Fig.3 Cycling performances,XRD and SEM of single-crystal NaNi0.3Fe0.4Mn0.3O2 after ethylene glycol or water rinsed[52]
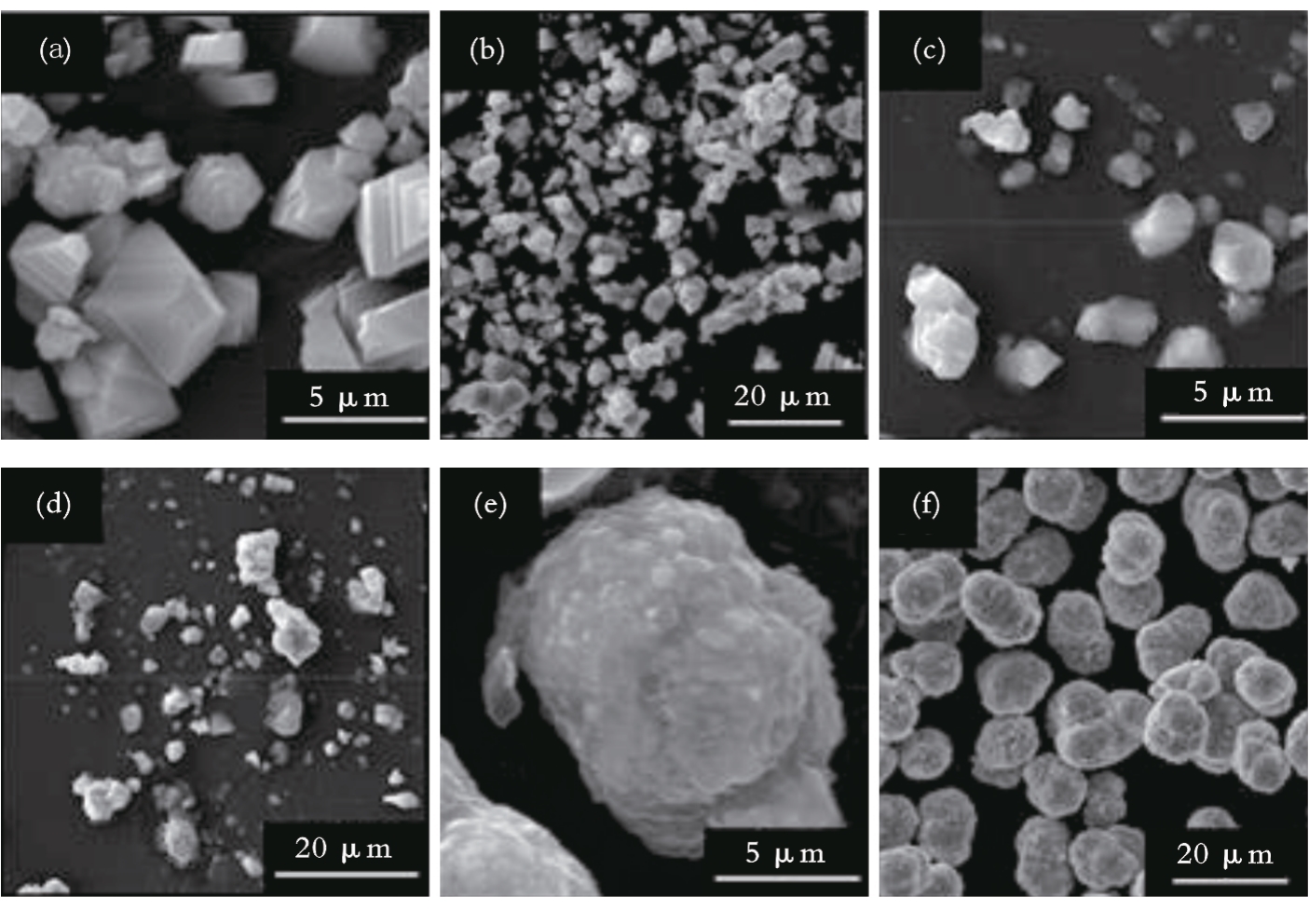
图5 水洗后中间混合相、单晶NaNi0.5Mn0.5O2和多晶NaNi0.5Mn0.5O2的SEM[57]注:(a)、(b)为水洗后中间混合相的SEM;(c)、(d)为单晶NaNi0.5Mn0.5O2的SEM;(e)、(f)为多晶NaNi0.5Mn0.5O2的SEM。
Fig.5 SEM of intermediate mixed phase, single-crystal NaNi0.5Mn0.5O2 and polycrystalline NaNi0.5Mn0.5O2 after water rinsed[57]
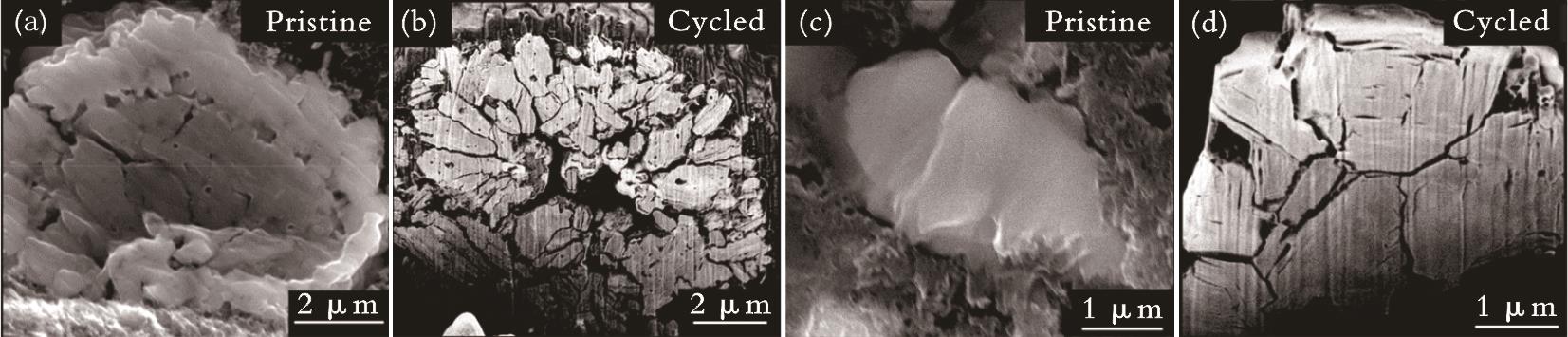
图6 多晶NaNi0.5Mn0.5O2和单晶NaNi0.5Mn0.5O2的SEM和循环200次后的FIB-SEM[57]注:(a)、(b)为多晶NaNi0.5Mn0.5O2的SEM和FIB-SEM;(c)、(d)为单晶NaNi0.5Mn0.5O2的SEM和FIB-SEM。
Fig.6 SEM and FIB-SEM after 200 cycles of polycrystalline NaNi0.5Mn0.5O2 and single-crystal NaNi0.5Mn0.5O2[57]
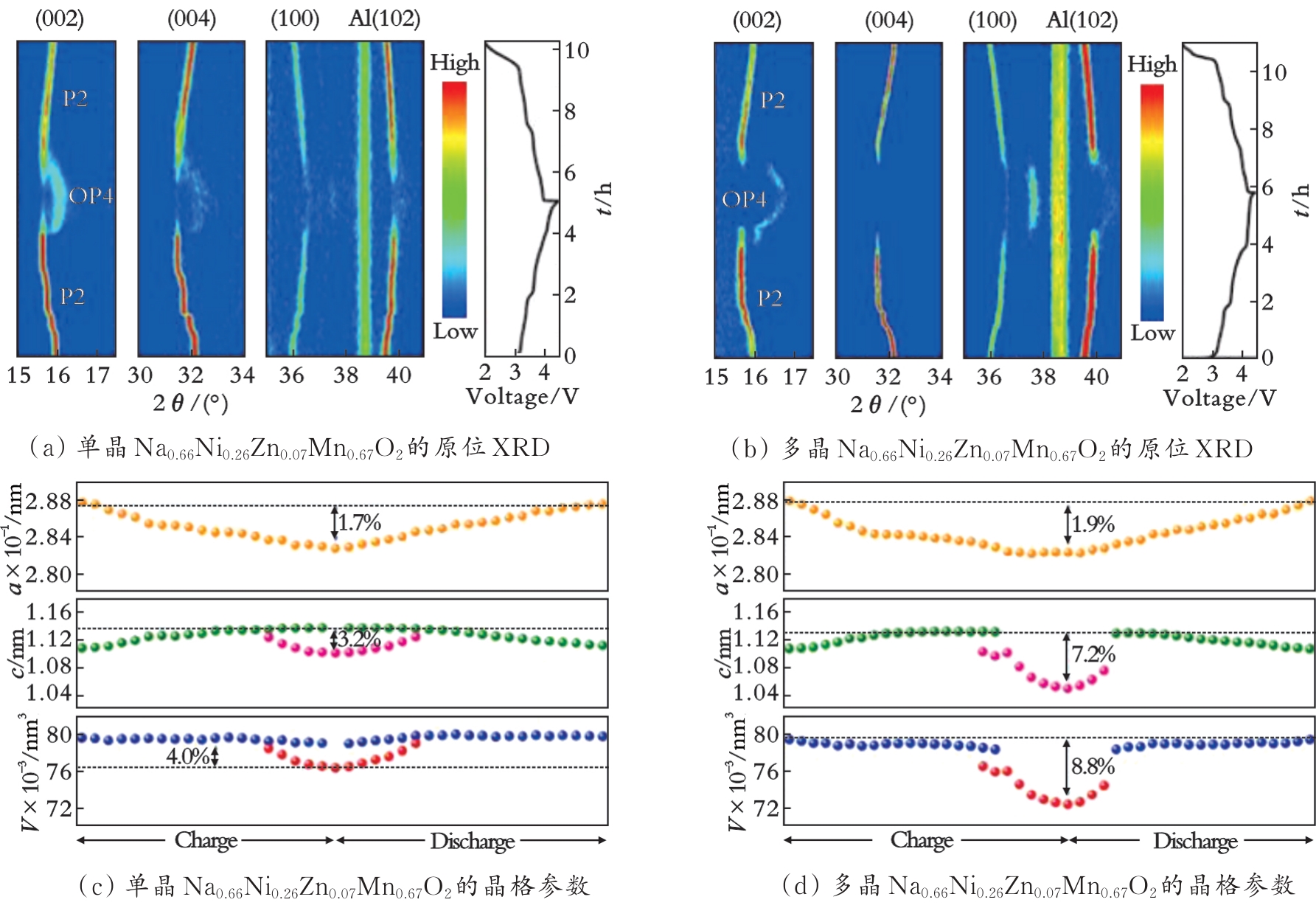
图7 P2型Na0.66Ni0.26Zn0.07Mn0.67O2的充放电原位XRD及其晶格参数曲线[53]
Fig.7 In situ XRD and lattice parameter curves of P2 type Na0.66Ni0.26Zn0.07Mn0.67O2 during charge and discharge[53]
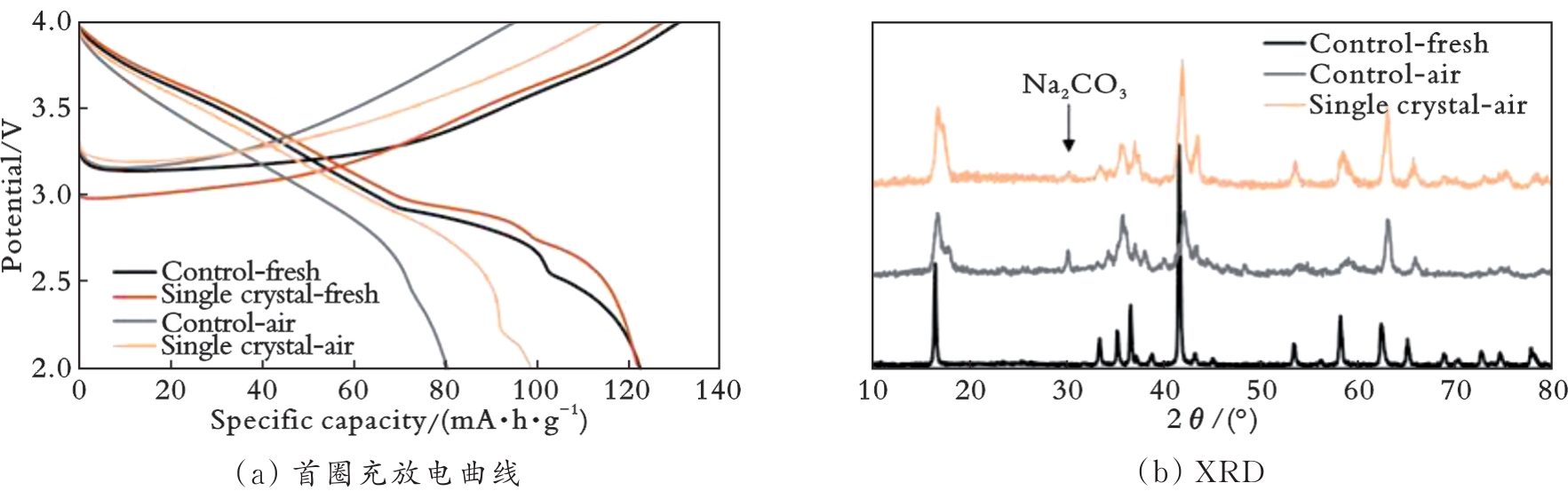
图8 单晶NaNi0.3Fe0.4Mn0.3O2和多晶NaNi0.3Fe0.4Mn0.3O2的空气稳定性对比[52]
Fig.8 Air stability of single-crystal NaNi0.3Fe0.4Mn0.3O2 versus polycrystal NaNi0.3Fe0.4Mn0.3O2[52]
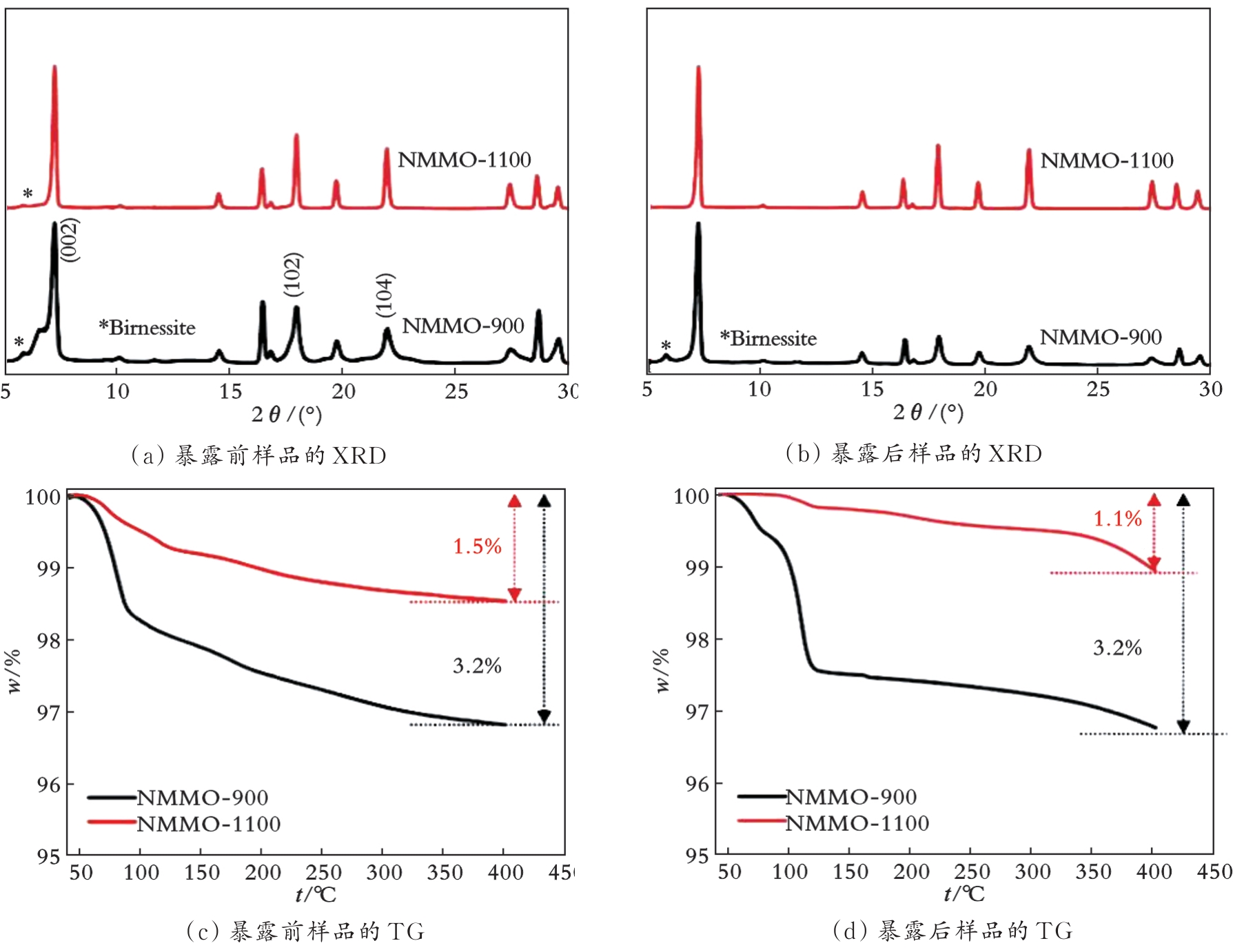
图9 空气暴露30 d前后单晶Na0.7Mn0.9Mg0.1O2和多晶Na0.7Mn0.9Mg0.1O2的空气稳定性对比[45]
Fig.9 Air stability comparison of single-crystal Na0.7Mn0.9Mg0.1O2 and polycrystalline Na0.7Mn0.9Mg0.1O2 before and after 30 days of air exposure[45]
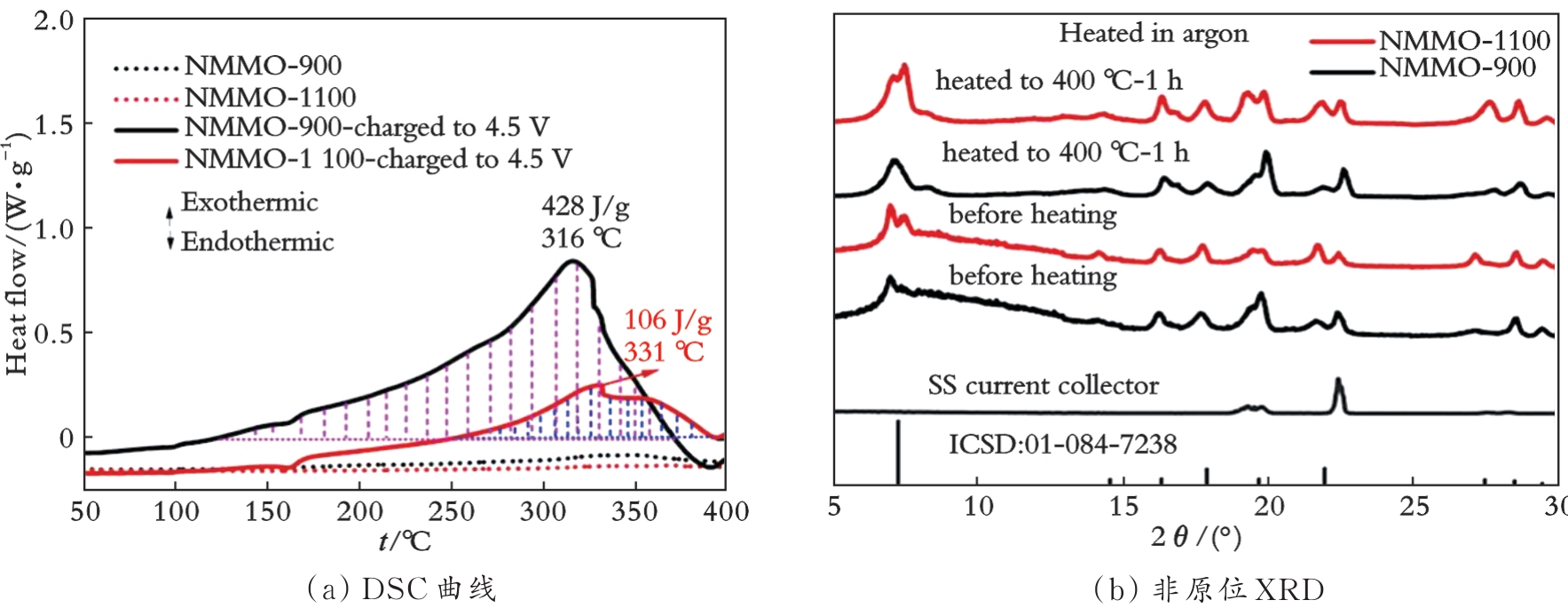
图10 单晶Na0.7Mn0.9Mg0.1O2和多晶Na0.7Mn0.9Mg0.1O2的热稳定性对比[45]
Fig.10 Comparison of thermal stability of single-crystal Na0.7Mn0.9Mg0.1O2 and polycrystalline Na0.7Mn0.9Mg0.1O2[45]
| 1 | KOMABA S, YABUUCHI N, NAKAYAMA T, et al. Study on the reversible electrode reaction of Na1- xNi0.5Mn0.5O2 for a rechargeable sodium-ion battery[J]. Inorganic Chemistry, 2012, 51(11): 6211-6220. |
| 2 | SATHIYA M, JACQUET Q, DOUBLET M L, et al. A chemical approach to raise cell voltage and suppress phase transition in O3 sodium layered oxide electrodes[J]. Advanced Energy Materials, 2018, 8(11): 1702599. |
| 3 | SHARMA N, GONZALO E, PRAMUDITA J C, et al. The unique structural evolution of the O3‐phase Na2/3Fe2/3Mn1/3O2 during high rate charge/discharge: A sodium-centred perspective[J]. Advanced Functional Materials, 2015, 25(31): 4994-5005. |
| 4 | WANG P F, YOU Y, YIN Y X, et al. Suppressing the P2-O2 phase transition of Na0.67 Mn0.67 Ni0.33 O2 by magnesium substitution for improved sodium-ion batteries[J]. Angewandte Chemie (International ed. in English), 2016, 55(26): 7445-7449. |
| 5 | XIE Y Y, WANG H, XU G L, et al. In operando XRD and TXM study on the metastable structure change of NaNi1/3Fe1/3Mn1/3O2 under electrochemical sodium-ion intercalation[J]. Advanced Energy Materials, 2016, 6(24): 1601306. |
| 6 | YU T Y, RYU H H, HAN G, et al. Understanding the capacity fading mechanisms of O3-type Na[Ni0.5Mn0.5]O2 cathode for sodium‐ion batteries[J]. Advanced Energy Materials, 2020, 10(37): 2001609. |
| 7 | 丁飞翔, 容晓晖, 王海波, 等. 钠离子层状氧化物材料相变及其对性能的影响[J]. 物理学报, 2022, 71(10): 1-24. |
| DING F X, RONG X H, WANG H B, et al. Phase transitions of Na-ion layered oxide materials and their influence on properties[J]. Acta Physica Sinica, 2022, 71(10): 1-24. | |
| 8 | 贺柳洋, 李晓杰, 王婵, 等. 石墨烯掺杂对三元钠离子电池正极材料电化学性能的影响[J]. 石油化工高等学校学报, 2023, 36(6): 64-72. |
| HE L Y, LI X J, WANG C, et al. Effect of graphene doping on electrochemical performance of cathode materials of ternary sodium-ion batteries[J]. Journal of Petrochemical Universities, 2023, 36(6): 64-72. | |
| 9 | 游济远, 曹永安, 孟绍良, 等. 钠离子电池正极材料研究进展[J]. 石油化工高等学校学报, 2022, 35(2): 1-8. |
| YOU J Y, CAO Y A, MENG S L, et al. Progress in cathode materials for sodium-ion batteries[J]. Journal of Petrochemical Universities, 2022, 35(2): 1-8. | |
| 10 | 刘欢庆, 高旭, 陈军, 等. 钠离子电池层状氧化物正极:层间滑移,相变与性能[J]. 储能科学与技术, 2020, 9(5): 1327-1339. |
| LIU H Q, GAO X, CHEN J, et al. Layered oxide cathode for sodium ion batteries: Interlayer glide, phase transition and performance[J]. Energy Storage Science and Technology, 2020, 9(5): 1327-1339. | |
| 11 | 张平, 康利斌, 王明菊, 等. 钠离子电池储能技术及经济性分析[J]. 储能科学与技术, 2022, 11(6): 1892-1901. |
| ZHANG P, KANG L B, WANG M J, et al. Technology feasibility and economic analysis of Na-ion battery energy storage[J]. Energy Storage Science and Technology, 2022, 11(6): 1892-1901. | |
| 12 | 胡海燕, 侴术雷, 肖遥. 基于分子轨道杂化的高电压钠离子电池层状氧化物正极材料[J]. 储能科学与技术, 2022, 11(4): 1093-1102. |
| HU H Y, HAO S L, XIAO Y. Layered oxide cathode materials based on molecular orbital hybridization for high voltage sodium-ion batteries[J]. Energy Storage Science and Technology, 2022, 11(4): 1093-1102. | |
| 13 | DUFFORT V, TALAIE E, BLACK R, et al. Uptake of CO2 in layered P2-Na0.67Mn0.5Fe0.5O2: Insertion of carbonate anions[J]. Chemistry of Materials, 2015, 27(7): 2515-2524. |
| 14 | LAMB J, MANTHIRAM A. Surface-modified Na(Ni0.3Fe0.4Mn0.3)O2 cathodes with enhanced cycle life and air stability for sodium-ion batteries[J]. ACS Applied Energy Materials, 2021, 4(10): 11735-11742. |
| 15 | LI X W, SHEN X, ZHAO J M, et al. O3-NaFe(1/3- x)Ni1/3Mn1/3AlxO2 cathodes with improved air stability for Na-ion batteries[J]. ACS Applied Materials & Interfaces, 2021, 13(28): 33015- 33023. |
| 16 | LU Z H, DAHN J R. Intercalation of water in P2, T2 and O2 structure Az[CoxNi1/3- xMn2/3]O2[J]. Chemistry of Materials, 2001, 13(4): 1252-1257. |
| 17 | 张浩然, 车海英, 郭凯强, 等. Sn掺杂NaNi1/3Fe1/3Mn1/3- xSnxO2正极材料制备及其电化学性能[J]. 储能科学与技术, 2022, 11(6): 1874-1882. |
| ZHANG H R, CHE H Y, GUO K Q, et al. Preparation of Sn-doped NaNi1/3Fe1/3Mn1/3- xSnxO2 cathode materials and their electrochemical performance[J]. Energy Storage Science and Technology, 2022, 11(6): 1874-1882. | |
| 18 | YUAN X G, GUO Y J, GAN L, et al. A universal strategy toward air-stable and high-rate O3 layered oxide cathodes for Na-ion batteries[J]. Advanced Functional Materials, 2022, 32(17): 2111466. |
| 19 | ZUO W H, LIU X S, QIU J M, et al. Engineering Na+-layer spacings to stabilize Mn-based layered cathodes for sodium-ion batteries[J]. Nature Communications, 2021, 12(1): 4903. |
| 20 | YOU Y, DOLOCAN A, LI W D, et al. Understanding the air-exposure degradation chemistry at a nanoscale of layered oxide cathodes for sodium-ion batteries[J]. Nano Letters, 2019, 19(1): 182-188. |
| 21 | SUN Y, WANG H, MENG D C, et al. Degradation mechanism of O3-type NaNi1/3Fe1/3Mn1/3O2 cathode materials during ambient storage and their in situ regeneration[J]. ACS Applied Energy Materials, 2021, 4(3): 2061-2067. |
| 22 | CHU S Y, ZHANG C C, XU H, et al. Pinning effect enhanced structural stability toward a zero-strain layered cathode for sodium-ion batteries[J]. Angewandte Chemie (International ed. in English), 2021, 60(24): 13366-13371. |
| 23 | GUO Y J, WANG P F, NIU Y B, et al. Boron-doped sodium layered oxide for reversible oxygen redox reaction in Na-ion battery cathodes[J]. Nature Communications, 2021, 12(1): 5267. |
| 24 | HWANG T, LEE J H, CHOI S H, et al. Critical role of titanium in O3-type layered cathode materials for sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2019, 11(34): 30894-30901. |
| 25 | JUNG K N, CHOI J Y, SHIN H S, et al. Mg-doped Na[Ni1/3Fe1/3Mn1/3]O2 with enhanced cycle stability as a cathode material for sodium-ion batteries[J]. Solid State Sciences, 2020, 106: 106334. |
| 26 | LENG M Z, BI J Q, WANG W L, et al. Superior electrochemical performance of O3-type NaNi0.5- xMn0.3Ti0.2ZrxO2 cathode material for sodium-ion batteries from Ti and Zr substitution of the transition metals[J]. Journal of Alloys and Compounds, 2020, 816: 152581. |
| 27 | SUN L Q, XIE Y Y, LIAO X Z, et al. Insight into Ca-substitution effects on O3-type NaNi1/3 Fe1/3Mn1/3O2 cathode materials for sodium-ion batteries application[J]. Small, 2018, 14(21): e1704523. |
| 28 | WANG C C, LIU L J, ZHAO S, et al. Tuning local chemistry of P2 layered-oxide cathode for high energy and long cycles of sodium-ion battery[J]. Nature Communications, 2021, 12(1): 2256. |
| 29 | HWANG J Y, MYUNG S T, CHOI J U, et al. Resolving the degradation pathways of the O3-type layered oxide cathode surface through the nano-scale aluminum oxide coating for high-energy density sodium-ion batteries[J]. Journal of Materials Chemistry A, 2017, 5(45): 23671-23680. |
| 30 | HWANG J Y, YU T Y, SUN Y K. Simultaneous MgO coating and Mg doping of Na[Ni0.5Mn0.5]O2 cathode: Facile and customizable approach to high-voltage sodium-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(35): 16854-16862. |
| 31 | LI N, REN J, DANG R B, et al. Suppressing phase transition and improving electrochemical performances of O3-NaNi1/3Mn1/3Fe1/3O2 through ionic conductive Na2SiO3 coating[J]. Journal of Power Sources, 2019, 429: 38-45. |
| 32 | LI N, WANG S F, ZHAO E Y, et al. Tailoring interphase structure to enable high-rate, durable sodium-ion battery cathode[J]. Journal of Energy Chemistry, 2022, 68: 564-571. |
| 33 | REN H X, ZHENG L M, LI Y, et al. Impurity-vibrational entropy enables quasi-zero-strain layered oxide cathodes for high-voltage sodium-ion batteries[J]. Nano Energy, 2022, 103(Part A): 107765. |
| 34 | SUN H H, HWANG J Y, YOON C S, et al. Capacity degradation mechanism and cycling stability enhancement of AlF3-coated nanorod gradient Na[Ni0.65Co0.08Mn0.27]O2 cathode for sodium-ion batteries[J]. ACS Nano, 2018, 12(12): 12912-12922. |
| 35 | WANG H B, DING F X, WANG Y Q, et al. In situ plastic-crystal-coated cathode toward high-performance Na-ion batteries[J]. ACS Energy Letters, 2023, 8(3): 1434-1444. |
| 36 | YU Y, KONG W J, LI Q Y, et al. Understanding the multiple effects of TiO2 coating on NaMn0.33Fe0.33Ni0.33O2 cathode material for Na-ion batteries[J]. ACS Applied Energy Materials, 2020, 3(1): 933-942. |
| 37 | 戚兴国, 王伟刚, 胡勇胜, 等. 钠离子电池层状氧化物正极材料的表面修饰研究[J]. 储能科学与技术, 2020, 9(5): 1396-1401. |
| QI X G, WANG W G, HU Y S, et al. Surface modification research of layered oxide materials for sodium-ion batteries[J]. Energy Storage Science and Technology, 2020, 9(5): 1396-1401. | |
| 38 | DUAN J G, WU C, CAO Y B, et al. Enhanced compacting density and cycling performance of Ni-riched electrode via building mono dispersed micron scaled morphology[J]. Journal of Alloys and Compounds, 2017, 695: 91-99. |
| 39 | BERTHELOT R, CARLIER D, DELMAS C. Electrochemical investigation of the P2–NaxCoO2 phase diagram[J]. Nature Materials, 2011, 10(1): 74-80. |
| 40 | HAN M H, GONZALO E, SINGH G, et al. A comprehensive review of sodium layered oxides: Powerful cathodes for Na-ion batteries[J]. Energy & Environmental Science, 2015, 8(1): 81-102. |
| 41 | ZHANG S Y, GUO Y J, ZHOU Y N, et al. P3/O3 integrated layered oxide as high-power and long-life cathode toward Na-ion batteries[J]. Small, 2021, 17(10): e2007236. |
| 42 | ZHOU Y N, WANG P F, NIU Y B, et al. A P2/P3 composite layered cathode for high-performance Na-ion full batteries[J]. Nano Energy, 2019, 55: 143-150. |
| 43 | LANGDON J, MANTHIRAM A. A perspective on single-crystal layered oxide cathodes for lithium-ion batteries[J]. Energy Storage Materials, 2021, 37: 143-160. |
| 44 | LI J, LI H Y, STONE W, et al. Synthesis of single crystal LiNi0.5Mn0.3Co0.2O2 for lithium ion batteries[J]. Journal of the Electrochemical Society, 2017, 164(14): A3529. |
| 45 | PAMIDI V, TRIVEDI S, BEHARA S, et al. Micron-sized single-crystal cathodes for sodium-ion batteries[J]. iScience, 2022, 25(5): 104205. |
| 46 | KIM Y. Lithium nickel cobalt manganese oxide synthesized using alkali chloride flux: Morphology and performance as a cathode material for lithium ion batteries[J]. ACS Applied Materials & Interfaces, 2012, 4(5): 2329-2333. |
| 47 | KIMIJIMA T, ZETTSU N, TESHIMA K. Growth manner of octahedral-shaped Li(Ni1/3Co1/3Mn1/3)O2 single crystals in molten Na2SO4[J]. Crystal Growth & Design, 2016, 16(5): 2618-2623. |
| 48 | LIANG R, WU Z Y, YANG W M, et al. A simple one-step molten salt method for synthesis of micron-sized single primary particle LiNi0.8Co0.1Mn0.1O2 cathode material for lithium-ion batteries[J]. Ionics, 2020, 26(4): 1635-1643. |
| 49 | QIAN G N, ZHANG Y T, LI L S, et al. Single-crystal nickel-rich layered-oxide battery cathode materials: Synthesis, electrochemistry, and intra-granular fracture[J]. Energy Storage Materials, 2020, 27: 140-149. |
| 50 | ZHU J, CHEN G Y. Single-crystal based studies for correlating the properties and high-voltage performance of Li-[NixMnyCo1- x- y]O2 cathodes[J]. Journal of Materials Chemistry A, 2019, 7(10): 5463-5474. |
| 51 | 任思佳, 田雷武, 邵钦君, 等. 助熔剂法制备单晶LiNi0.8Co0.1Mn0.1O2正极材料[J]. 储能科学与技术, 2020, 9(6): 1702-1713. |
| REN S J, TIAN L W, SHAO Q J, et al. Synthesis of single-crystal LiNi0.8Co0.1Mn0.1O2 by flux method[J]. Energy Storage Science and Technology, 2020, 9(6): 1702-1713. | |
| 52 | LAMB J, JARVIS K, MANTHIRAM A. Molten-salt synthesis of O3-type layered oxide single crystal cathodes with controlled morphology towards long-life sodium-ion batteries[J]. Small, 2022, 18(43): 2106927. |
| 53 | ZHANG F P, LU Y, GUO Y, et al. Highly stabilized single-crystal P2-type layered oxides obtained via rational crystal orientation modulation for sodium-ion batteries[J]. Chemical Engineering Journal, 2023, 458: 141515. |
| 54 | LI F, KONG L L, SUN Y Y, et al. Micron-sized monocrystalline LiNi1/3Co1/3Mn1/3O2 as high-volumetric-energy-density cathode for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2018, 6(26): 12344-12352. |
| 55 | LI H Y, LI J, ZAKER N, et al. Synthesis of single crystal LiNi0.88Co0.09Al0.03O2 with a two-step lithiation method[J]. Journal of the Electrochemical Society, 2019, 166(10): A1956. |
| 56 | LIU A, ZHANG N, STARK J E, et al. Synthesis of Co-free Ni-rich single crystal positive electrode materials for lithium ion batteries: Part I. Two-step lithiation method for Al-or Mg-doped LiNiO2[J]. Journal of the Electrochemical Society, 2021, 168(4): 040531. |
| 57 | DARGA J, MANTHIRAM A. Facile synthesis of O3-type NaNi0.5Mn0.5O2 single crystals with improved performance in sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2022, 14(47): 52729-52737. |
| 58 | ZHANG L, HUANG J Y, SONG M Y, et al. Single-crystal growth of P2-type layered oxides with increased exposure of{010}planes for high-performance sodium-ion batteries[J]. ACS Applied Materials & Interfaces, 2023, 15(40): 47037-47048. |
| 59 | KONAROV A, KIM H J, JO J H, et al. High-voltage oxygen-redox-based cathode for rechargeable sodium-ion batteries[J]. Advanced Energy Materials, 2020, 10(24): 2001111. |
| 60 | KUMAKURA S, TAHARA Y, KUBOTA K, et al. Sodium and manganese stoichiometry of P2-type Na2/3MnO2[J]. Angewandte Chemie (International ed. in English), 2016, 55(41): 12760-12763. |
| 61 | ZUO W H, QIU J M, LIU X S, et al. The stability of P2-layered sodium transition metal oxides in ambient atmospheres[J]. Nature Communications, 2020, 11(1): 3544. |
| 62 | LIU Q, LIU Y T, ZHAO C, et al. Conformal PEDOT coating enables ultra-high-voltage and high-temperature operation for single-crystal Ni-rich cathodes[J]. ACS Nano, 2022, 16(9): 14527-14538. |
| 63 | SHEN J X, ZHANG B, HUANG W Y, et al. Achieving thermodynamic stability of single-crystal Co-free Ni-rich cathode material for high voltage lithium-ion batteries[J]. Advanced Functional Materials, 2023, 33(23): 2300081. |
| 64 | SONG Y J, CUI Y P, LI B Y, et al. Revealing the origin of high-thermal-stability of single-crystal Ni-rich cathodes toward higher-safety batteries[J]. Nano Energy, 2023, 116: 108846. |
| 65 | LIU X, XU G L, KOLLURU V S C, et al. Origin and regulation of oxygen redox instability in high-voltage battery cathodes[J]. Nature Energy, 2022, 7(9): 808-817. |
| 66 | ZHANG H L, LIU H, PIPER L F J, et al. Oxygen loss in layered oxide cathodes for Li-ion batteries: Mechanisms, effects, and mitigation[J]. Chemical Reviews, 2022, 122(6): 5641-5681. |
| 67 | ZHANG S S. Problems and their origins of Ni-rich layered oxide cathode materials[J]. Energy Storage Materials, 2020, 24: 247-254. |
| 68 | HUANG Y H. The discovery of cathode materials for lithium-ion batteries from the view of interdisciplinarity[J]. Interdisciplinary Materials, 2022, 1(3): 323-329. |
| 69 | ZHANG L X, LIU Y M, YOU Y, et al. NASICONs-type solid-state electrolytes: The history, physicochemical properties, and challenges[J]. Interdisciplinary Materials, 2023, 2(1): 91-110. |
| [1] | 魏婷婷, 王振宏, 伊廷锋. 钠离子电池层状正极材料相变与电荷补偿机制[J]. 石油化工高等学校学报, 2024, 37(6): 13-24. |
| [2] | 王新宇, 张凯洋, 者荣杰, 刘汉浩, 谷振一, 何晓燕, 吴兴隆. 钠离子电池煤基硬碳负极材料的改性制备与应用挑战[J]. 石油化工高等学校学报, 2024, 37(6): 25-34. |
| [3] | 赵昊, 刘香楠, 王静怡, 吕佳璇, 王翔, 郎笑石, 蔡克迪. 钠离子电池正极材料研究进展[J]. 石油化工高等学校学报, 2024, 37(6): 35-43. |
| [4] | 吕士忠, 李天龙, 房睿, 卢宝光, 郑成志, 赵磊, 邓亮, 王振波. 磷酸钒钠正极材料的Zr4+掺杂改性研究[J]. 石油化工高等学校学报, 2024, 37(6): 44-51. |
| [5] | 张博杨, 滕彦梅. O3型层状过渡金属氧化物正极材料高电压稳定性研究[J]. 石油化工高等学校学报, 2024, 37(6): 52-61. |
| [6] | 刘海燕, 苑仁鲁, 龙厚坤, 沈祺, 赵博洋, 刘学伟, 宋怀河. 纤维素衍生硬炭的结构调控和储钠性能研究[J]. 石油化工高等学校学报, 2024, 37(6): 62-73. |
| [7] | 赵恩锋, 武宏大, 蔡天凤, 杨占旭. 钠离子电池电解液添加剂的研究进展[J]. 石油化工高等学校学报, 2024, 37(5): 65-72. |
| [8] | 盛大伟, DIN HASEEB UD, 张济原, 刘晓旭. 还原氧化石墨烯微观结构调控及储钠性能研究[J]. 石油化工高等学校学报, 2023, 36(6): 48-56. |
| [9] | 贺柳洋, 李晓杰, 王婵, 段春阳. 石墨烯掺杂对三元钠离子电池正极材料电化学性能的影响[J]. 石油化工高等学校学报, 2023, 36(6): 64-72. |
| [10] | 宋翰林, 吕雪川, 张晓帆, 张雅琦, 李成龙, 高肖汉. 稀土(铕,钕)⁃2,6⁃吡啶二甲酸配合物的合成、结构及其性质[J]. 石油化工高等学校学报, 2023, 36(1): 58-65. |
| [11] | 张博, 郄佳鑫, 曹永安, 赵久成, 吴军, 王文举. 基于可视化分析辅助探究钠离子电池硬碳负极研究进展[J]. 石油化工高等学校学报, 2022, 35(6): 1-9. |
| [12] | 游济远, 曹永安, 孟绍良, 赵久成, 吴军, 王文举. 钠离子电池正极材料研究进展[J]. 石油化工高等学校学报, 2022, 35(2): 1-8. |
| [13] | 沙畅畅, 毛杨杨, 曹永安, 王文举. 用于锂硫电池正极的生物质碳材料制备与应用[J]. 石油化工高等学校学报, 2020, 33(3): 1-7. |
| [14] | 杨占旭,乔庆东,康晓雪,姜海波. 高温固相法制备LiFePO4/C 正极材料及其性能研究[J]. 石油化工高等学校学报, 2011, 24(5): 38-40. |
| [15] | 杨占旭,乔庆东. 锂离子电池正极材料LiNi0.8Co0.2O2的合成及性能研究[J]. 石油化工高等学校学报, 2011, 24(1): 18-20. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
网站版权 © 2024《石油化工高等学校学报》编辑部
地址:辽宁省抚顺市望花区丹东路西段1号 电话:024-56860967 E-mail:lnxuebao@126.com 邮编:113001
本系统由北京玛格泰克科技发展有限公司设计开发