


石油化工高等学校学报 ›› 2025, Vol. 38 ›› Issue (3): 20-31.DOI: 10.12422/j.issn.1006-396X.2025.03.003
温涛1( ), 李小成1, 凡纪鹏1, 邓一坤2, 邹菁1, 王海涛1(
), 李小成1, 凡纪鹏1, 邓一坤2, 邹菁1, 王海涛1( )
)
收稿日期:2024-06-22
修回日期:2024-07-17
出版日期:2025-06-26
发布日期:2025-07-02
通讯作者:
王海涛
作者简介:温涛(1998⁃),男,硕士研究生,从事锂离子电池正极材料的制备及性能方面的研究;E⁃mail:18094040551@163.com。
基金资助:
Tao WEN1( ), Xiaocheng LI1, Jipeng FAN1, Yikun DENG2, Jing ZOU1, Haitao WANG1(
), Xiaocheng LI1, Jipeng FAN1, Yikun DENG2, Jing ZOU1, Haitao WANG1( )
)
Received:2024-06-22
Revised:2024-07-17
Published:2025-06-26
Online:2025-07-02
Contact:
Haitao WANG
摘要:
高镍三元正极材料LiNi x Co y Mn1-x-y O2(x≥0.6,NCM)由于其成本低廉、能量密度高、使用寿命长等优势,被认为是最具应用价值的锂离子电池正极材料之一。高镍虽然会显著提升NCM的比容量和能量密度,但也会导致其循环和热稳定性下降,因此其实际应用严重受限。对NCM进行掺杂改性是提升材料结构稳定性、改善其电化学性能的有效策略。详细介绍了NCM材料的掺杂方法;系统分析了多种掺杂元素对NCM容量、倍率性能、循环性能等的影响;对NCM的开发和未来所面临的挑战进行了展望,有望为NCM的应用提供参考。
中图分类号:
温涛, 李小成, 凡纪鹏, 邓一坤, 邹菁, 王海涛. 高镍锂离子电池三元正极材料的掺杂改性研究进展[J]. 石油化工高等学校学报, 2025, 38(3): 20-31.
Tao WEN, Xiaocheng LI, Jipeng FAN, Yikun DENG, Jing ZOU, Haitao WANG. Research Progress on Doping Modification of Ternary Cathode Materials for Nickel⁃Rich Lithium Ion Batteries[J]. Journal of Petrochemical Universities, 2025, 38(3): 20-31.
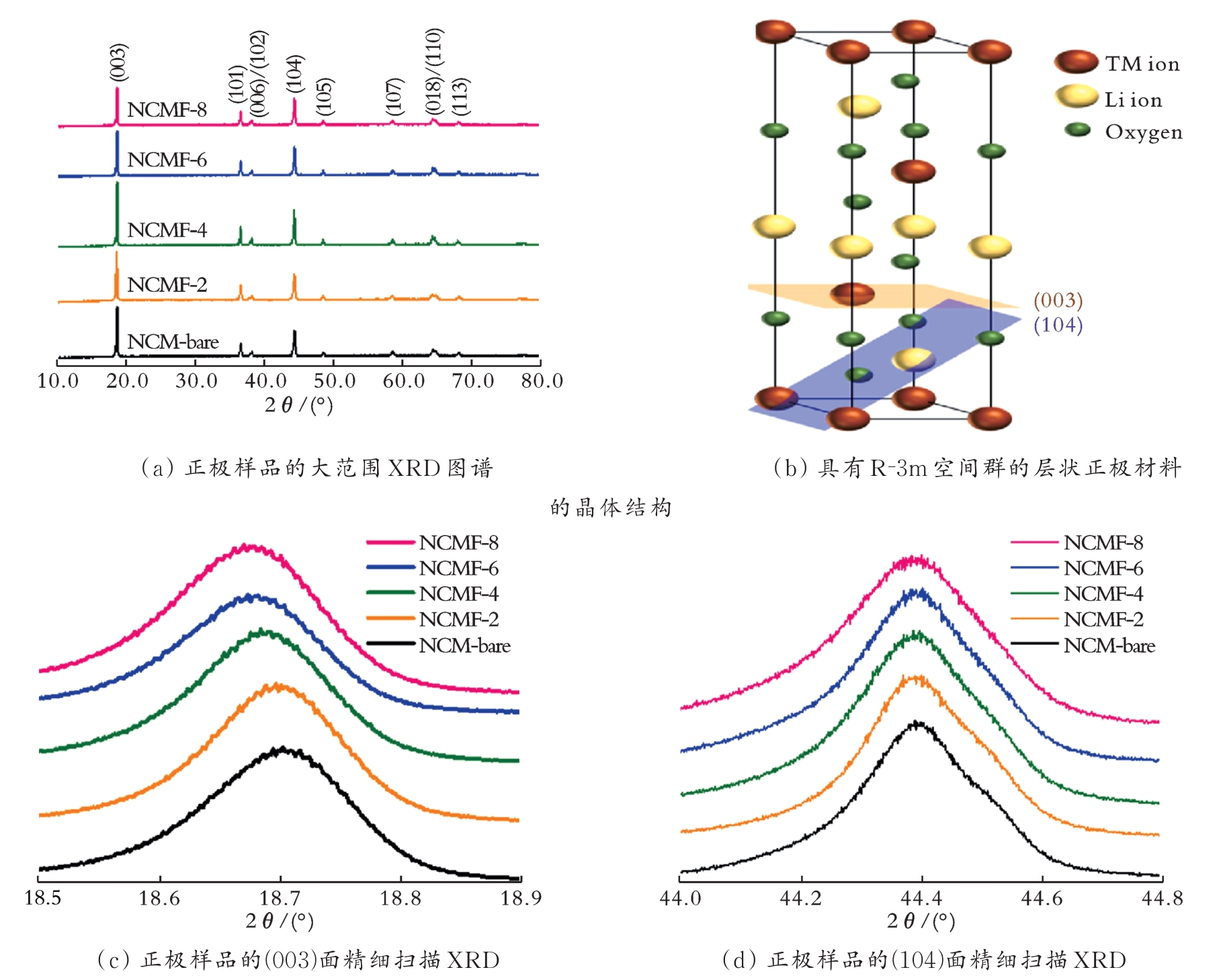
图2 正极样品的大范围XRD图谱、具有R-3m空间群的层状正极材料的晶体结构及正极样品的(003)和(104)面精细扫描XRD[20]
Fig.2 Wide-range XRD patterns of the cathode samples and crystal structure of a layered cathode material with anR-3m space group and fine-scan XRD patterns in the (003) and (104) planes of the cathode samples[20]
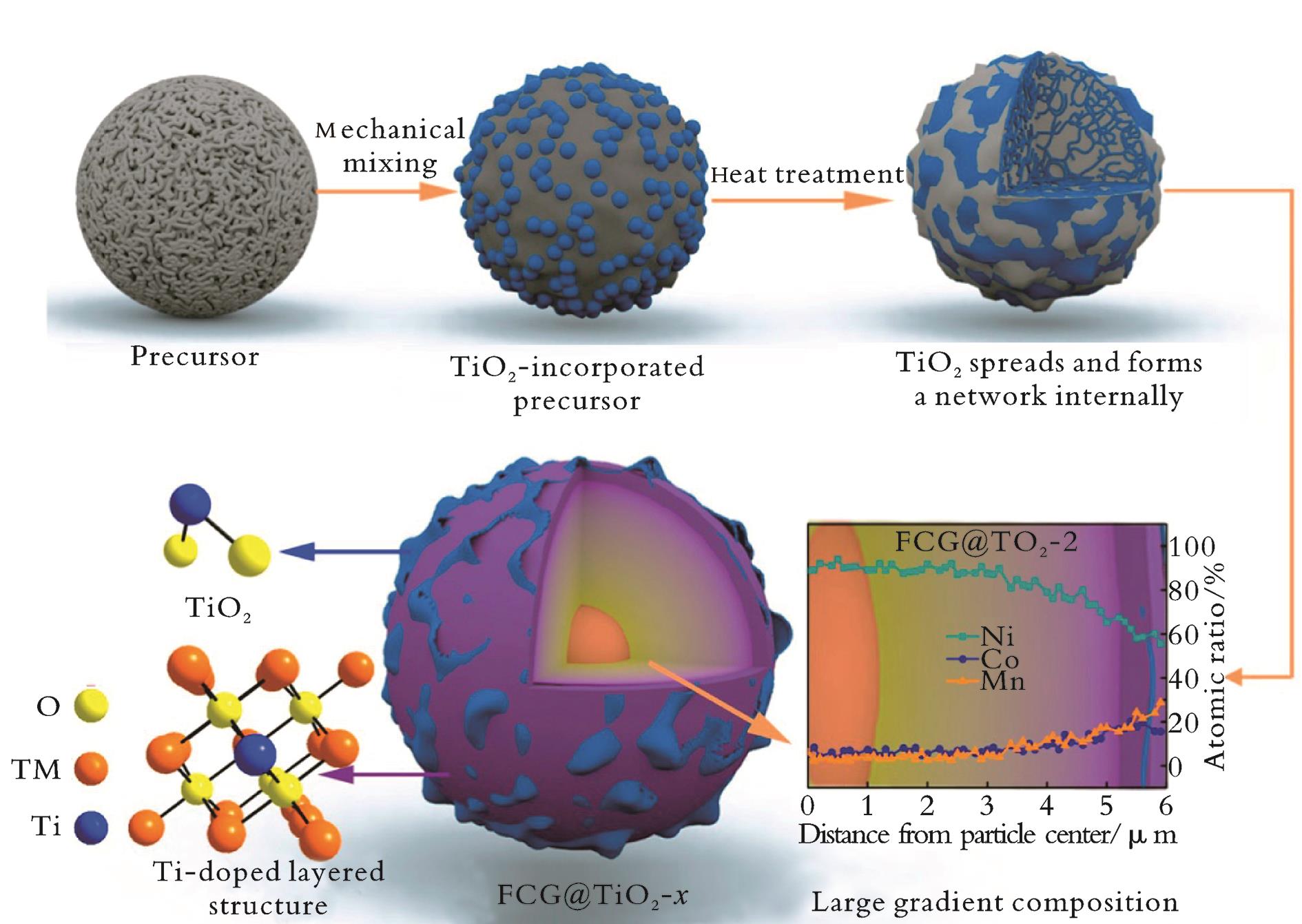
图5 FCG@TiO2-x 微球合成路线示意图及循环200次后微观形貌[31]
Fig.5 Schematic illustration of the synthetic route to FCG@TiO2-x and microspheres micro-topography after 200 cycles[31]
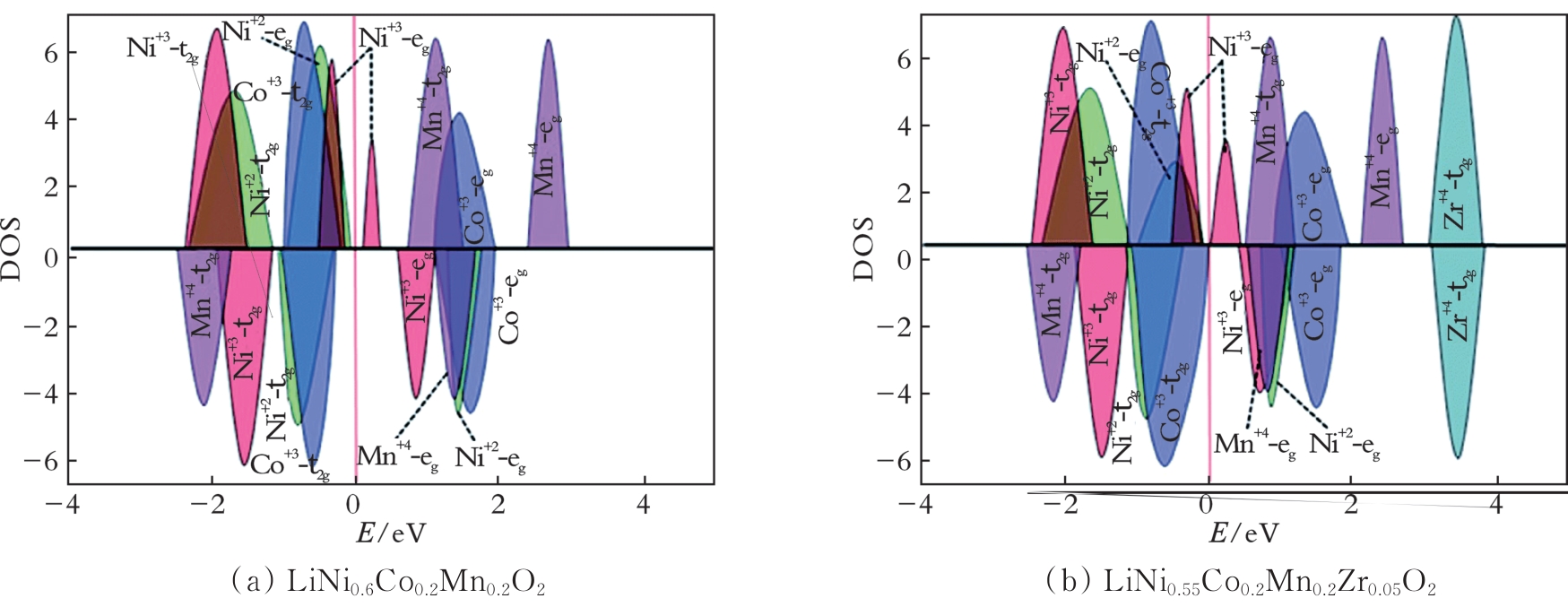
图6 LiNi0.6Co0.2Mn0.2O2和LiNi0.55Co0.2Mn0.2Zr0.05O2的PDOS投影图[37]
Fig.6 Schematic projected density of states (PDOS) for LiNi0.6Co0.2Mn0.2O2 and LiNi0.55Co0.2Mn0.2Zr0.05O2[37]
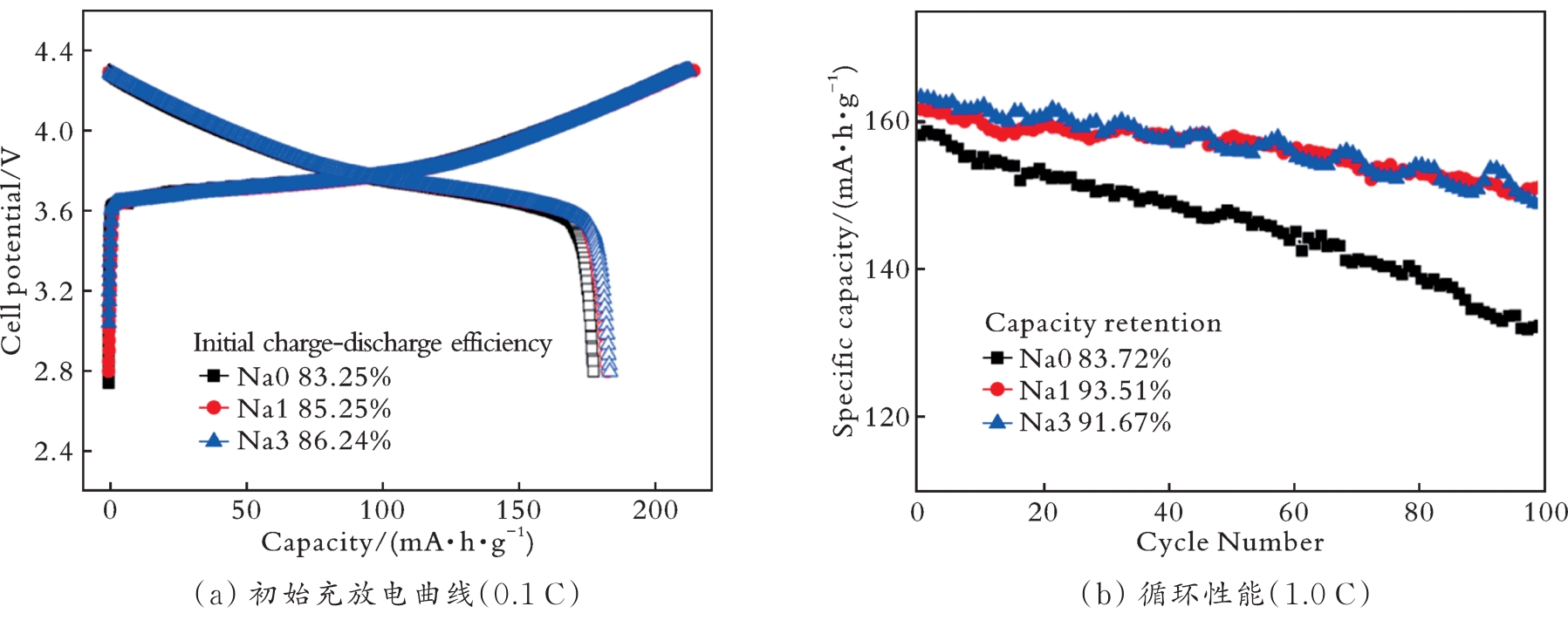
图9 样品在2.8~4.3 V、恒流密度下的初始充放电曲线和循环性能[52]
Fig.9 Initial charge/discharge curves and cycle performance of the samples at a constant current density of 2.8~4.3 V[52]
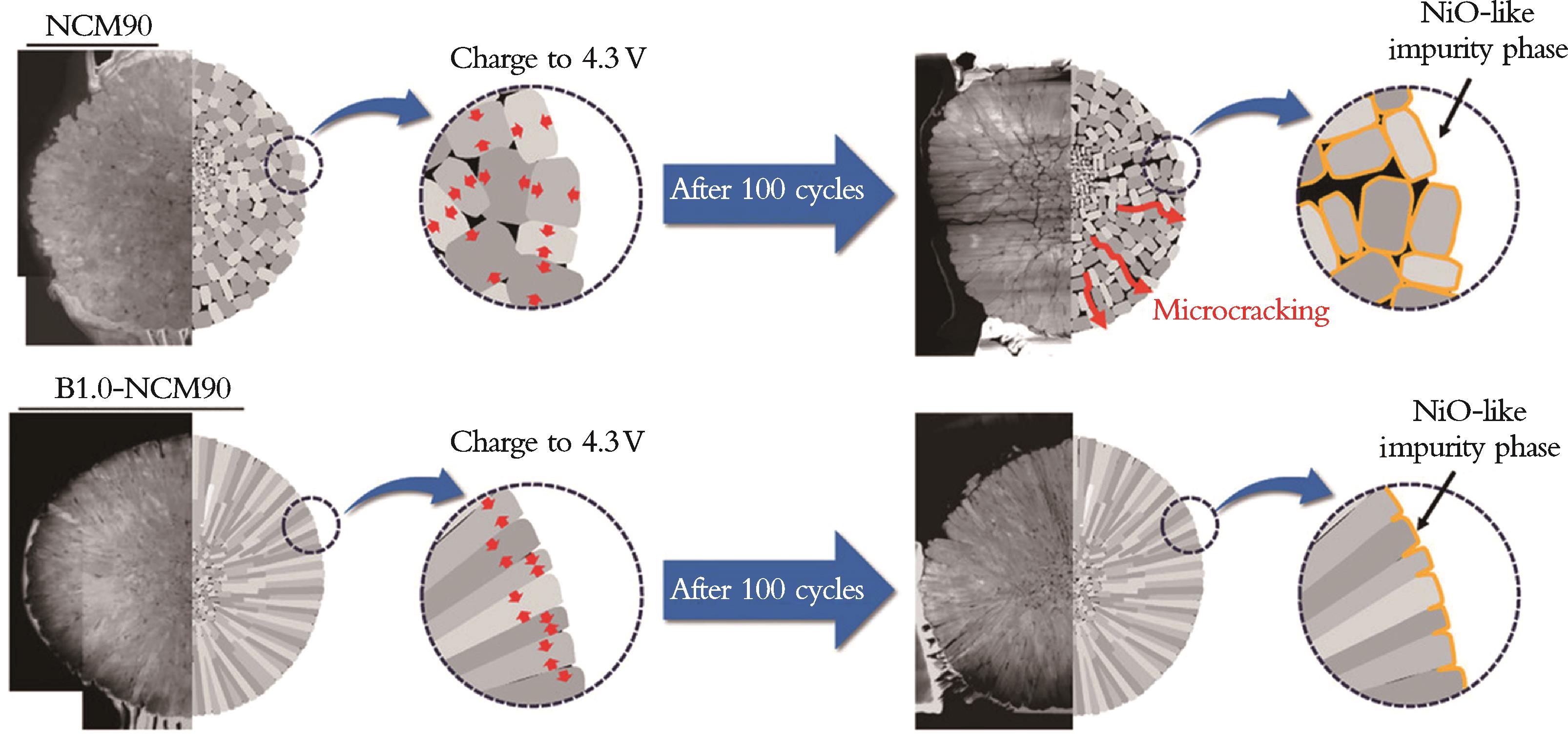
图10 充放电循环过程中B掺杂对NCM90正极机械稳定性影响的示意图[66]
Fig.10 Schematic illustration of the effect of boron-doping on the NCM90 cathode's mechanical stability during charge and discharge cycling[66]
| 掺杂元素 | 放电容量/(mA·h·g-1) | 循环性能 | 作用机理 | 文献 | ||
|---|---|---|---|---|---|---|
| 初始样 | 改性样 | 初始样 | 改性样 | |||
| Ti | 188.0(0.5 C) | 196.0(0.5 C) | 循环100次后容量保持率为70.0%(1.0 C) | 循环100次后容量保持率为84.0%(1.0 C) | Ti4+为NMC811晶格稳定性提供了额外的支持 | [ |
| Zr | 178.3(2.0 C) | 189.4(2.0 C) | 循环200次后容量保持率为77.8%(1.0 C) | 循环200次后容量保持率为79.5%(1.0 C) | Zr4+的加入使NMC811的结构稳定,内应力降低,保持颗粒结构的相对完整性 | [ |
| Nb | 148.8(1.0 C) | 181.6(1.0 C) | 循环100次后容量保持率为81.8%(1.0 C) | 循环100次后容量保持率为94.6%(1.0 C) | Nb修饰的固体边界表面足以抵抗HF和有害物质对活性物质的毒性作用,减少极化现象 | [ |
| Na | 173.0(0.1 C) | 186.0(0.1 C) | 循环100次后容量保持率为83.7%(1.0 C) | 循环100次后容量保持率为93.5%(1.0 C) | Na+的掺杂减少了阳离子的混合,同时稳定了层间结构 | [ |
| Mg | 148.0(0.5 C) | 155.0(0.5 C) | 循环30次后容量保持率为90.7%(0.5 C) | 循环30次后容量保持率为95.8%(0.5 C) | Mg取代Co提高了Li+的扩散速率 | [ |
| B | 230.0(0.1 C) | 237.0(0.1 C) | 循环100次后容量保持率为76.0%(0.1 C) | 循环100次后容量保持率为91.0%(0.1 C) | 硼离子对可以部分缓解NCM90正极深度充电过程中产生的固有内部应变 | [ |
| F | 179.0(0.5 C) | 201.0(0.5 C) | 循环50次后容量保持率为63.0%(0.5 C) | 循环50次后容量保持率为88.0%(0.5 C) | 用F-取代氧可以在过渡金属和F之间产生较强的键,使晶格参数c增大 | [ |
表1 元素掺杂的作用效果以及作用机理
Table 1 The effect and mechanism of element doping
| 掺杂元素 | 放电容量/(mA·h·g-1) | 循环性能 | 作用机理 | 文献 | ||
|---|---|---|---|---|---|---|
| 初始样 | 改性样 | 初始样 | 改性样 | |||
| Ti | 188.0(0.5 C) | 196.0(0.5 C) | 循环100次后容量保持率为70.0%(1.0 C) | 循环100次后容量保持率为84.0%(1.0 C) | Ti4+为NMC811晶格稳定性提供了额外的支持 | [ |
| Zr | 178.3(2.0 C) | 189.4(2.0 C) | 循环200次后容量保持率为77.8%(1.0 C) | 循环200次后容量保持率为79.5%(1.0 C) | Zr4+的加入使NMC811的结构稳定,内应力降低,保持颗粒结构的相对完整性 | [ |
| Nb | 148.8(1.0 C) | 181.6(1.0 C) | 循环100次后容量保持率为81.8%(1.0 C) | 循环100次后容量保持率为94.6%(1.0 C) | Nb修饰的固体边界表面足以抵抗HF和有害物质对活性物质的毒性作用,减少极化现象 | [ |
| Na | 173.0(0.1 C) | 186.0(0.1 C) | 循环100次后容量保持率为83.7%(1.0 C) | 循环100次后容量保持率为93.5%(1.0 C) | Na+的掺杂减少了阳离子的混合,同时稳定了层间结构 | [ |
| Mg | 148.0(0.5 C) | 155.0(0.5 C) | 循环30次后容量保持率为90.7%(0.5 C) | 循环30次后容量保持率为95.8%(0.5 C) | Mg取代Co提高了Li+的扩散速率 | [ |
| B | 230.0(0.1 C) | 237.0(0.1 C) | 循环100次后容量保持率为76.0%(0.1 C) | 循环100次后容量保持率为91.0%(0.1 C) | 硼离子对可以部分缓解NCM90正极深度充电过程中产生的固有内部应变 | [ |
| F | 179.0(0.5 C) | 201.0(0.5 C) | 循环50次后容量保持率为63.0%(0.5 C) | 循环50次后容量保持率为88.0%(0.5 C) | 用F-取代氧可以在过渡金属和F之间产生较强的键,使晶格参数c增大 | [ |
| 1 | XIA Y,ZHENG J M,WANG C M,et al.Designing principle for Ni⁃rich cathode materials with high energy density for practical applications[J].Nano Energy,2018,49:434⁃452. |
| 2 | ZHANG S S.Understanding of performance degradation of LiNi0.80Co0.10Mn0.10O2 cathode material operating at high potentials[J].Journal of Energy Chemistry,2020,41:135⁃141. |
| 3 | KIM J,LEE H,CHA H,et al.Prospect and reality of Ni‐rich cathode for commercialization[J].Advanced Energy Materials,2018,8(6):1702028. |
| 4 | MANTHIRAM A,SONG B H,LI W D.A perspective on nickel⁃rich layered oxide cathodes for lithium⁃ion batteries[J].Energy Storage Materials,2017,6:125⁃139. |
| 5 | XIAO B W,SUN X L.Surface and subsurface reactions of lithium transition metal oxide cathode materials:An overview of the fundamental origins and remedying approaches[J].Advanced Energy Materials,2018,8(29):1802057. |
| 6 | DIXIT M,MARKOVSKY B,SCHIPPER F,et al.Origin of structural degradation during cycling and low thermal stability of Ni⁃rich layered transition metal⁃based electrode materials[J].The Journal of Physical Chemistry C,2017,121(41):22628⁃22636. |
| 7 | DO S J,SANTHOSHKUMAR P,KANG S H,et al.Al⁃doped Li[Ni0.78Co0.1Mn0.1Al0.02]O2 for high performance of lithium ion batteries[J].Ceramics International,2019,45(6):6972⁃6977. |
| 8 | MARKUS I M,LIN F,KAM K C,et al.Computational and experimental investigation of Ti substitution in Li⁃ (NixMnxCo1-2 x-yTiy) O2 for lithium ion batteries[J].The Journal of Physical Chemistry Letters,2014,5(21):3649⁃3655. |
| 9 | ZHU H W,YU H F,JIANG H B,et al.High⁃efficiency Mo doping stabilized LiNi0.9Co0.1O2 cathode materials for rapid charging and long⁃life Li⁃ion batteries[J].Chemical Engineering Science,2020,217:115518. |
| 10 | GAO S,ZHAN X W,CHENG Y T.Structural,electrochemical and Li⁃ion transport properties of Zr⁃modified LiNi0.8Co0.1Mn0.1O2 positive electrode materials for Li⁃ion batteries[J].Journal of Power Sources,2019,410⁃411:45⁃52. |
| 11 | YU H F,ZHU H W,YANG Z F,et al.Bulk Mg⁃doping and surface polypyrrole⁃coating enable high⁃rate and long⁃life for Ni⁃rich layered cathodes[J].Chemical Engineering Journal,2021,412:128625. |
| 12 | TAO J L,MU A N,GENG S J,et al.Influences of direction and magnitude of Mg2+ doping concentration gradient on the performance of full concentration gradient cathode material[J].Journal of Solid State Electrochemistry,2021,25(7):1959⁃1974. |
| 13 | KIM U H,LEE E J,YOON C S,et al.Compositionally graded cathode material with long‐term cycling stability for electric vehicles application[J].Advanced Energy Materials,2016,6(22):1601417. |
| 14 | KONG D F,HU J T,CHEN Z F,et al.Ti‐gradient doping to stabilize layered surface structure for high performance high‐Ni oxide cathode of Li‐ion battery[J].Advanced Energy Materials,2019,9(41):1901756. |
| 15 | LI H,XU Q,SHI X X,et al.Electrochemical performance of LiNi0.5Mn0.5O2 with different synthesis methods[J].Rare Metals,2015,34(8):580⁃585. |
| 16 | YAO X,XU Z M,YAO Z Y,et al.Oxalate co⁃precipitation synthesis of LiNi0.6Co0.2Mn0.2O2 for low⁃cost and high⁃energy lithium⁃ion batteries[J].Materials Today Communications,2019,19:262⁃270. |
| 17 | YANG W T,LI R F,CHEN Y,et al.Preparation of a high performance LiNi0.6Co0.2Mn0.2O2 cathode material by using citric acid as a complexing agent[J].Green Chemistry,2023,25(3):1085⁃1095. |
| 18 | VAN WESTEN T,GROOT R D.Effect of temperature cycling on ostwald ripening[J].Crystal Growth & Design,2018,18(9):4952⁃4962. |
| 19 | PROSINI P P,MANCINI R,PETRUCCI L,et al.Li4Ti5O12 as anode in all⁃solid⁃state,plastic,lithium⁃ion batteries for low⁃power applications[J].Solid State Ionics,2001,144(1/2):185⁃192. |
| 20 | KIM S B,KIM H,PARK D H,et al.Li⁃ion diffusivity and electrochemical performance of Ni⁃rich cathode material doped with fluoride ions[J].Journal of Power Sources,2021,506:230219. |
| 21 | WANG T,REN K L,HE M,et al.Synthesis and manipulation of single⁃crystalline lithium nickel manganese cobalt oxide cathodes:A review of growth mechanism[J].Frontiers in Chemistry,2020,8:747. |
| 22 | ZHU Y J,CHOI S H,FAN X L,et al.Recent progress on spray pyrolysis for high performance electrode materials in lithium and sodium rechargeable batteries[J].Advanced Energy Materials,2017,7(7):1601578. |
| 23 | SONG S A,PARK S B,HAN J.Synthesis of zirconium⁃based material⁃coated LiNi0.8Co0.2O2 cathode using a new coating method[J].Japanese Journal of Applied Physics,2012,51(10R):105202. |
| 24 | YOU B Z,SUN J P,JING Y,et al.A fresh one⁃step spray pyrolysis approach to prepare nickel⁃rich cathode material for lithium⁃ion batteries[J].ACS Applied Materials & Interfaces,2023,15(11):14587⁃14595. |
| 25 | AHN W,LIM S N,JUNG K N,et al.Combustion⁃synthesized LiNi0.6Mn0.2Co0.2O2 as cathode material for lithium ion batteries[J].Journal of Alloys and Compounds,2014,609:143⁃149. |
| 26 | XU L P,ZHOU F,LIU B,et al.Progress in preparation and modification of LiNi0.6Mn0.2Co0.2O2 cathode material for high energy density Li⁃ion batteries[J].International Journal of Electrochemistry,2018,2018:6930386. |
| 27 | CHENG Y,SUN Y,CHU C T,et al.Stabilizing effects of atomic Ti doping on high⁃voltage high⁃nickel layered oxide cathode for lithium⁃ion rechargeable batteries[J].Nano Research,2022,15(5):4091⁃4099. |
| 28 | SUN H B,CAO Z L,WANG T R,et al.Enabling high rate performance of Ni⁃rich layered oxide cathode by uniform titanium doping[J].Materials Today Energy,2019,13:145⁃151. |
| 29 | DU R,BI Y J,YANG W C,et al.Improved cyclic stability of LiNi0.8Co0.1Mn0.1O2 via Ti substitution with a cut⁃off potential of 4.5 V[J].Ceramics International,2015,41(5,Part B):7133⁃7139. |
| 30 | KONG D F,HU J T,CHEN Z F,et al.Ti⁃gradient doping to stabilize layered surface structure for high performance high⁃Ni oxide cathode of Li⁃ion battery[J].Advanced Energy Materials,2019,9(41):1901756. |
| 31 | MO Y,GUO L J,JIN H F,et al.Building nickel⁃rich cathodes with large concentration gradient for high performance lithium⁃ion batteries[J].Journal of Power Sources,2020,468:228405. |
| 32 | PARK K,HAM D J,PARK S Y,et al.High⁃Ni cathode material improved with Zr for stable cycling of Li⁃ion rechargeable batteries[J].RSC Advances,2020,10(45):26756⁃26764. |
| 33 | LI L Y,HAN Y,ZHAO B B,et al.Enhancing the cycle stability of Zr⁃doped LiNi0.83Co0.12Mn0.05O2 by co⁃precipitation[J].Ionics,2022,28(3):1037⁃1046. |
| 34 | HAN B,XU S,ZHAO S,et al.Enhancing the structural stability of Ni⁃rich layered oxide cathodes with a preformed Zr⁃concentrated defective nanolayer[J].ACS Applied Materials & Interfaces,2018,10(46):39599⁃39607. |
| 35 | LI X,ZHANG K J,WANG M S,et al.Dual functions of zirconium modification on improving the electrochemical performance of Ni⁃rich LiNi0.8Co0.1Mn0.1O2[J].Sustainable Energy & Fuels,2018,2(2):413⁃421. |
| 36 | FENG Z,HUANG X B,RAJAGOPALAN R,et al.Enhanced electrochemical properties of LiNi0.8Co0.1Mn0.1O2 at elevated temperature by simultaneous structure and interface regulating[J].Journal of the Electrochemical Society,2019,166(8):A1439. |
| 37 | SCHIPPER F,DIXIT M,KOVACHEVA D,et al.Stabilizing nickel⁃rich layered cathode materials by a high⁃charge cation doing strategy:Zirconium⁃doped LiNi0.6Co0.2Mn0.2O2[J].Journal of Materials Chemistry A,2016,4(41):16073⁃16084. |
| 38 | LEVARTOVSKY Y,CHAKRABORTY A,KUNNIKURUVAN S,et al.Enhancement of structural,electrochemical,and thermal properties of high⁃energy density Ni⁃rich LiNi0.85Co0.1Mn0.05O2 cathode materials for li⁃ion batteries by niobium doping[J].ACS Applied Materials & Interfaces,2021,13(29):34145⁃34156. |
| 39 | LI J,ZHANG M L,ZHANG D Y,et al.An effective doping strategy to improve the cyclic stability and rate capability of Ni⁃rich LiNi0.8Co0.1Mn0.1O2 cathode[J].Chemical Engineering Journal,2020,402:126195. |
| 40 | SUN C C,CHEN W X,GAO P,et al.Investigation of structure and cycling performance of Nb⁃doped nickel⁃rich single⁃crystal ternary cathode materials[J].Ionics,2022,28(2):747⁃757. |
| 41 | KANEDA H,KOSHIKA Y,NAKAMURA T,et al.Improving the cycling performance and thermal stability of LiNi0.6Co0.2Mn0.2O2 cathode materials by Nb⁃doping and surface modification[J].International Journal of Electrochemical Science,2017,12(6):4640⁃4653. |
| 42 | LEVARTOVSKY Y,CHAKRABORTY A,KUNNIKURUVAN S,et al.Enhancement of structural,electrochemical,and thermal properties of high⁃energy density Ni⁃rich LiNi0.85Co0.1Mn0.05O2 cathode materials for Li⁃ion batteries by niobium doping[J].ACS Applied Materials & Interfaces,2021,13(29):34145⁃34156. |
| 43 | CHU B B,LIU S Y,YOU L Z,et al.Enhancing the cycling stability of Ni⁃rich LiNi0.6Co0.2Mn0.2O2 cathode at a high cutoff voltage with Ta doping[J].ACS Sustainable Chemistry & Engineering,2020,8(8):3082⁃3090. |
| 44 | ZOU Y G,MAO H C,MENG X H,et al.Mitigating the kinetic hindrance of single‐crystalline Ni‐rich cathode via surface gradient penetration of tantalum[J].Angewandte Chemie International Edition,2021,133(51):26739⁃26743. |
| 45 | RAJKAMAL A,KIM H.Formation of pillar⁃ions in the Li layer decreasing the Li/Ni disorder and improving the structural stability of cation⁃doped Ni⁃rich LiNi0.8Co0.1Mn0.1O2:A first⁃principles verification[J].ACS Applied Energy Materials,2021,4(12):14068⁃14079. |
| 46 | ZHANG R H,ZHENG Y D,YAO Z Y,et al.Systematic study of Al impurity for NCM622 cathode materials[J].ACS Sustainable Chemistry & Engineering,2020,8(26):9875⁃9884. |
| 47 | AURBACH D,SRUR⁃LAVI O,GHANTY C,et al.Studies of aluminum⁃doped LiNi0.5Co0.2Mn0.3O2:Electrochemical behavior,aging,structural transformations,and thermal characteristics[J].Journal of the Electrochemical Society,2015,162(6):A1014. |
| 48 | LU Y,MO Y,CHEN Y,et al.Effects of various elements doping on LiNi0.6Co0.2Mn0.2O2 layered materials for lithium‐ion batteries[J].Energy Technology,2021,9(7):2100074. |
| 49 | EILERS⁃RETHWISCH M,HILDEBRAND S,EVERTZ M,et al.Comparative study of Sn⁃doped Li[Ni0.6Mn0.2Co0.2- xSnx]O2 cathode active materials(x=0~0.5) for lithium ion batteries regarding electrochemical performance and structural stability[J].Journal of Power Sources,2018,397:68⁃78. |
| 50 | WANG L,LIANG J S,ZHANG X Y,et al.An effective dual⁃modification strategy to enhance the performance of LiNi0.6Co0.2Mn0.2O2 cathode for Li⁃ion batteries[J].Nanoscale,2021,13(8):4670⁃4677. |
| 51 | GONG C X,LU W X,QU L M,et al.Syntheses and electrochemical properties of layered Li0.95Na0.05Ni1/3Co1/3Mn1/3O2 and LiNi1/3Co1/3Mn1/3O2[J].Journal of Power Sources,2014,247:151⁃155. |
| 52 | HUANG Z J,WANG Z X,JING Q,et al.Investigation on the effect of Na doping on structure and Li⁃ion kinetics of layered LiNi0.6Co0.2Mn0.2O2 cathode material[J].Electrochimica Acta,2016,192:120⁃126. |
| 53 | ZHAO R R,YANG Z L,LIANG J X,et al.Understanding the role of Na⁃doping on Ni⁃rich layered oxide LiNi0.5Co0.2Mn0.3O2[J].Journal of Alloys and Compounds,2016,689:318⁃325. |
| 54 | SHEN Y B,YAO X J,ZHANG J H,et al.Sodium doping derived electromagnetic center of lithium layered oxide cathode materials with enhanced lithium storage[J].Nano Energy,2022,94:106900. |
| 55 | SUN Y X,ZHANG L J,ZHOU Y,et al.Study on potassium doped modification of Li1.2Ni0.13Co0.13Mn0.54O2 materials synthesized by novel method for lithium ion battery[J].Journal of the Electrochemical Society,2018,165(2):A333. |
| 56 | HE T,CHEN L,SU Y F,et al.The effects of alkali metal ions with different ionic radii substituting in Li sites on the electrochemical properties of Ni⁃rich cathode materials[J].Journal of Power Sources,2019,441:227195. |
| 57 | XU T T,LIU C,GUO Z X,et al.Improved rate and cyclic performance of potassium⁃doped nickel⁃rich ternary cathode material for lithium⁃ion batteries[J].Journal of Materials Science,2021,56(3):2399⁃2411. |
| 58 | FU C Y,ZHOU Z L,LIU Y H,et al.Synthesis and electrochemical properties of Mg⁃doped LiNi0.6Co0.2Mn0.2O2 cathode materials for Li⁃ion battery[J].Journal of Wuhan University of Technology⁃Mater.Sci.Ed.,2011,26(2):211⁃215. |
| 59 | HUANG Z J,WANG Z X,ZHENG X B,et al.Effect of Mg doping on the structural and electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials[J].Electrochimica Acta,2015,182:795⁃802. |
| 60 | LIAO P Y,DUH J G,SHEU H S.Structural and thermal properties of LiNi0.6- xMgxCo0.25Mn0.15O2 cathode materials[J].Journal of Power Sources,2008,183(2):766⁃770. |
| 61 | WOO S W,MYUNG S T,BANG H,et al.Improvement of electrochemical and thermal properties of Li[Ni0.8Co0.1Mn0.1]O2 positive electrode materials by multiple metal (Al,Mg) substitution[J].Electrochimica Acta,2009,54(15):3851⁃3856. |
| 62 | CHEN M M,ZHAO E Y,CHEN D F,et al.Decreasing Li/Ni disorder and improving the electrochemical performances of Ni⁃rich LiNi0.8Co0.1Mn0.1O2 by Ca doping[J].Inorganic Chemistry,2017,56(14):8355⁃8362. |
| 63 | WANG Y Y,SONG X,LIU S,et al.Elucidating the effect of the dopant Ionic radius on the structure and electrochemical performance of Ni⁃rich layered oxides for lithium⁃ion batteries[J].ACS Applied Materials & Interfaces,2021,13(47):56233⁃56241. |
| 64 | RAJKAMAL A,KIM H.Formation of pillar⁃ions in the Li layer decreasing the Li/Ni disorder and improving the structural stability of cation⁃doped Ni⁃rich LiNi0.8Co0.1Mn0.1O2:A first⁃principles verification[J].ACS Applied Energy Materials,2021,4(12):14068⁃14079. |
| 65 | JAMIL S,WANG G,YANG L,et al.Suppressing H2–H3 phase transition in high Ni–low Co layered oxide cathode material by dual modification[J].Journal of Materials Chemistry A,2020,8(40):21306⁃21316. |
| 66 | PARK K J,JUNG H G,KUO L Y,et al.Improved cycling stability of Li[Ni0.9Co0.05Mn0.05]O2 through microstructure modification by boron doping for Li‐ion batteries[J].Advanced Energy Materials,2018,8(25):1801202. |
| 67 | ZHANG N,LI Y,QIAO Y F.Boosting the electrochemical performance of LiNi0.6Mn0.2Co0.2O2 through a trace amount of Mg⁃B co⁃doping[J].Journal of Materials Science & Technology,2021,89:167⁃178. |
| 68 | ROITZHEIM C,KUO L Y,SOHN Y J,et al.Boron in Ni⁃rich NCM811 cathode material:Impact on atomic and microscale properties[J].ACS Applied Energy Materials,2021,5(1):524⁃538. |
| 69 | NATION L,WU Y,JAMES C,et al.Si⁃doped high⁃energy Li1.2Mn0.54Ni0.13Co0.13O2 cathode with improved capacity for lithium⁃ion batteries[J].Journal of Materials Research,2018,33(24):4182⁃4191. |
| 70 | WEIGEL T,SCHIPPER F,ERICKSON E M,et al.Structural and electrochemical aspects of LiNi0.8Co0.1Mn0.1O2 cathode materials doped by various cations[J].ACS Energy Letters,2019,4(2):508⁃516. |
| 71 | LIANG C P,KONG F T,LONGO R C,et al.Site⁃dependent multicomponent doping strategy for Ni⁃rich LiNi1-2 yCoyMnyO2(y=1/12)cathode materials for Li⁃ion batteries[J].Journal of Materials Chemistry A,2017,5(48):25303⁃25313. |
| 72 | KIM G H,KIM M H,MYUNG S T,et al.Effect of fluorine on Li[Ni1/3Co1/3Mn1/3]O2- zFz as lithium intercalation material[J].Journal of Power Sources,2005,146(1⁃2):602⁃605. |
| 73 | KIM H,KIM S B,PARK D H,et al.Fluorine⁃doped LiNi0.8Mn0.1Co0.1O2 cathode for high⁃performance lithium⁃ion batteries[J].Energies,2020,13(18):4808. |
| 74 | QIU Q Q,YUAN S S,BAO J,et al.Suppressing irreversible phase transition and enhancing electrochemical performance of Ni⁃rich layered cathode LiNi0.9Co0.05Mn0.05O2 by fluorine substitution[J].Journal of Energy Chemistry,2021,61:574⁃581. |
| 75 | SI Z,SHI B Z,HUANG J,et al.Titanium and fluorine synergetic modification improves the electrochemical performance of Li(Ni0.8Co0.1Mn0.1)O2[J].Journal of Materials Chemistry A,2021,9(14):9354⁃9363. |
| 76 | ZENG J B,SHEN Y,REN X F,et al.F⁃doped Ni⁃rich layered cathode material with improved rate performance for lithium⁃ion batteries[J].Processes,2022,10(8):1573. |
| 77 | PARK J,PARK S,BEAK M,et al.Impacts of residual electrolyte components of spent lithium⁃ion batteries on the physical/electrochemical properties of resynthesized cathode active materials[J].Journal of Cleaner Production,2022,379(Part 1):134570. |
| 78 | GAO S,WANG L J,ZHOU C Y,et al.In⁃situ construction protective layer and phosphate doping synergistically improve the long⁃term cycle stability of LiNi0.6Co0.1Mn0.3O2[J].Chemical Engineering Journal,2021,426:131359. |
| 79 | FAN X P,TAN C L,LI Y,et al.A green,efficient,closed⁃loop direct regeneration technology for reconstructing of the LiNi0.5Co0.2Mn0.3O2 cathode material from spent lithium⁃ion batteries[J].Journal of Hazardous Materials,2021,410:124610. |
| 80 | TANG H,FU W W,XIE T,et al.High performance of phosphorus and fluorine co⁃doped nickel⁃rich cathode material for lithium ion batteries[J].Solid State Ionics,2021,361:115550. |
| [1] | 刘畅, 王彦淇, 周佰洵, 卓文祺, 王振波. 钠离子电池硬碳负极材料的研究进展:从材料设计到电化学性能优化[J]. 石油化工高等学校学报, 2025, 38(3): 1-9. |
| [2] | 沈雨可, 李欢, 马紫峰, 李林森. 钠离子电池硬碳负极材料的孔结构表征方法综述[J]. 石油化工高等学校学报, 2025, 38(3): 10-19. |
| [3] | 张文武, 张熊, 李晨, 孙现众, 王凯, 马向东. 金属单原子在钠离子电容器中的应用进展[J]. 石油化工高等学校学报, 2025, 38(1): 1-10. |
| [4] | 贺东玮, 张彬, 尤雅. 钠离子单晶层状氧化物正极研究进展[J]. 石油化工高等学校学报, 2024, 37(6): 1-12. |
| [5] | 吕士忠, 李天龙, 房睿, 卢宝光, 郑成志, 赵磊, 邓亮, 王振波. 磷酸钒钠正极材料的Zr4+掺杂改性研究[J]. 石油化工高等学校学报, 2024, 37(6): 44-51. |
| [6] | 马文钊, 王利娟. 焦磷酸锡负极材料的制备及性能研究[J]. 石油化工高等学校学报, 2024, 37(4): 76-82. |
| [7] | 贺柳洋, 李晓杰, 王婵, 段春阳. 石墨烯掺杂对三元钠离子电池正极材料电化学性能的影响[J]. 石油化工高等学校学报, 2023, 36(6): 64-72. |
| [8] | 赵鹤鸣, 陈丽萍, 魏奇, 于龙娇, 杨健松, 石富强, 王世伟. 钙钛矿光伏电池封装材料的制备与性能研究[J]. 石油化工高等学校学报, 2023, 36(5): 67-72. |
| [9] | 宋学实, 曲微丽, 赵磊, 王振波. 质子交换膜燃料电池氧还原Pt基催化剂研究进展[J]. 石油化工高等学校学报, 2023, 36(4): 25-33. |
| [10] | 冯莲晶, 王利娟. Sn4P3⁃G@C负极在锂离子电池中的应用[J]. 石油化工高等学校学报, 2023, 36(1): 66-73. |
| [11] | 刘盈汐, 王吉林, 王璐璐, 曲志强. 基于植酸框架构筑燃料电池用阴离子导电膜[J]. 石油化工高等学校学报, 2023, 36(1): 74-80. |
| [12] | 李磊, 王昆彦, 王育乔. 阳离子取代对尖晶石型硫化物储能性能的影响[J]. 石油化工高等学校学报, 2022, 35(6): 59-65. |
| [13] | 唐波, 刘乾锋, 樊贺飞, 张强, 王二东. 不同金属基底改性Zn负极的电化学性能研究[J]. 石油化工高等学校学报, 2022, 35(6): 66-73. |
| [14] | 杨扬, 孟超, 胡涵, 吴明铂. NiCo2O4/Ru复合催化剂的制备及锌空电池的应用[J]. 石油化工高等学校学报, 2022, 35(5): 64-70. |
| [15] | 沈紫烨, 王利娟. 高比容量Li2ZnTi3O8@C⁃N负极材料储锂性能研究[J]. 石油化工高等学校学报, 2022, 35(3): 1-9. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
网站版权 © 2024《石油化工高等学校学报》编辑部
地址:辽宁省抚顺市望花区丹东路西段1号 电话:024-56860967 E-mail:lnxuebao@126.com 邮编:113001
本系统由北京玛格泰克科技发展有限公司设计开发